New Delhi, Sep 23 (IANS) Researchers at the Institute of Nano Science and Technology (INST), Mohali, an autonomous institute of Department of Science and Technology (DST) have developed catalytic droplets that can lead to faster chemical reactions and help accelerate drug development and lower healthcare costs.
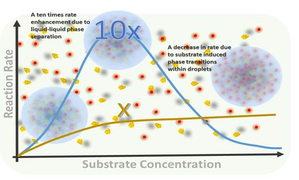
The catalytic droplets can lead to a 10-fold increase in speed and efficient catalytic reactions and this is valid below a critical substrate concentration, said the researchers.
“Such efficient chemical reactions can accelerate drug development, leading to quicker access to innovative medications and potentially lower healthcare costs,” revealed the findings, published in the journal Nanoscale.
The team led by Professor Sarmistha Sinha and colleagues explored a way to confine nano-catalyst molecules without impeding their movement.
They sought to confine protein-metal nanocomposites within droplets formed through liquid-liquid phase separation.
Unlike traditional methods, which relied on physical and chemical barriers to confine molecules during catalytic reactions, the new approach allowed for barrier-free confinement, so that the molecules within the droplets could move freely.
The droplets themselves were indifferent to the native conformation of the proteins they contained, creating an ideal environment for catalysis. The result was a staggering 10-fold increase in the catalytic efficiency of the metal nanocatalysts.
This discovery opens up new possibilities for accelerating chemical reactions, making them faster and more efficient than ever before.
The finding published in the journal Nanoscale represents a paradigm shift in approach to chemical reactions. The ability to confine molecules within barrier-free droplets while maintaining — or even amplifying reaction rates could lead to more efficient industrial processes, from drug manufacturing to energy production.
Moreover, the insights gained from understanding phase transitions within these droplets could pave the way for new technologies that harness the power of liquid-liquid phase separation, said the researchers.
–IANS
rvt/

Comments are closed.